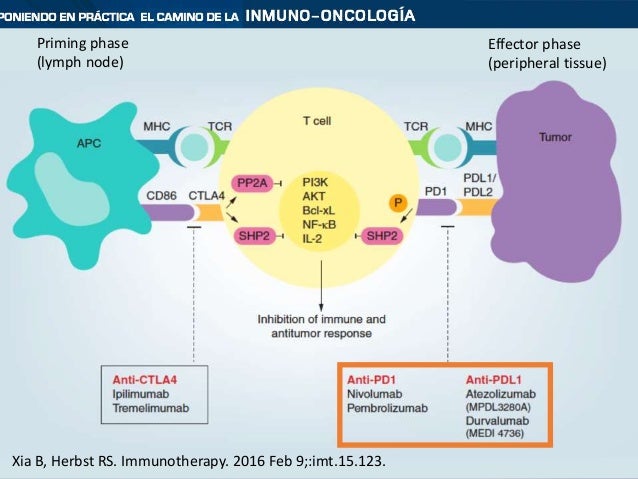
It is in the clinical trials where the differences start to show. Comparison of os was estimated using an inverse probability weighting model to reduce bias between the groups.
Keytruda is a type of immunotherapy that helps boost the immune system�s response against cancer.
Difference between opdivo and keytruda. These drugs help your immune system detect cancer cells in your body and stop their growth. Additionally, although both keytruda and opdivo are approved for 9 indications as of the end of 2016, the differences in the underlying patient populations for each of these indications makes it difficult which drug has a larger. Keytruda and opdivo, there is really no difference between the two drugs.
Keytruda did give better results in recent clinical trials, but then it sounds like the participants were more carefully selected than in the opdivo trials. It is in the clinical trials where the differences start to show. That preference has played out in sales.
Considering the pressure that keytruda’s growing number of favorable data bring to competitors, these two people are completely understandable. But this is like saying i purposely chose a small cohort with a greater probability of highlighting my drug’s. While keytruda revenues grew an impressive 62% compared to q3 2018 to reach $3.1m, opdivo sales increased only by 1% compared to q3 2018 to $1.8m.
Overall, these drugs are great. The orr was significantly higher in the pembrolizumab group than in the nivolumab group, while pfs was not significantly different between the two groups. Opdivo is an immune checkpoint inhibitor that may be used to treat many different cancers including melanoma, liver cancer, and lung cancer.
These may, for example, be due to differences between the patient populations in the clinical trials designed to test the drugs. Merck may have more specific patient inclusion criteria so that keytruda shows better efficacy compared to bms’ efforts with opdivo. From the societal perspective, the difference is substantial.
There�s been no head to head comparison so i wouldn�t put a whole lot of emphasis on the slight edge keytruda seems to have so far. Keytruda is a type of immunotherapy that helps boost the immune system�s response against cancer. How else would you choose between the two agents when deciding on treatment for a patient?
However, opdivo’s first approval was in september of 2014, while keytruda has only ben on market since late 2015. Keytruda seems to have a slight edge but very slight. Statistically there�s not much of a difference between opdivo and keytruda.
There are many people here on inspire who have achieved ned or stability with opdivo. Opdivo and keytruda may be used alone. There are many factors that are the reason for these differences.
This article explains what are the differences between keytruda and opdivo. The one that requires less testing, opdivo, had $2.1 billion in 2015 sales versus keytruda�s $566 million. In comparison, roche’s triplet reduced the risk of death.
Comparison of os was estimated using an inverse probability weighting model to reduce bias between the groups. The difference becomes many thousands of dollars throughout the course of treatment for a single patient. They have basically equivalent response rates and side effect profiles.
The financial impact of this difference is significant, with potential savings of approximately $2000 per dose. Learn more about immunotherapies for breast cancer. Merck may have more specific patient inclusion criteria so that keytruda shows better efficacy compared to bms’ efforts with opdivo.
Several immunotherapy medicines are approved by the fda to treat breast cancer, including herceptin, perjeta, and kadcyla (immune targeted therapies), and keytruda and tecentriq (immune checkpoint inhibitors). There are many factors that are the reason for these differences. It is in the clinical trials where the differences start to show.
Oncologists for previously treated, unresectable or metastatic malignant. Immunotherapy medicines work by helping your immune system attack cancer cells. But this is like saying i purposely chose a small cohort with a greater probability of highlighting my drug’s.
It may be used to treat many different types of cancer in adults and children and is given by.