
Orlando (march 12, 2018) — the experimental drug andexanet was associated with control of serious bleeding in patients taking a common class of anticoagulants known as factor xa inhibitors, according to interim clinical trial results presented at the american college of cardiology’s 67th annual scientific session. Total plasma clearance values were 884 and 1212 ml/h/kg in rats and dogs, respectively.
And sales of each drug.
Factor xa inhibitor drugs. Orlando (march 12, 2018) — the experimental drug andexanet was associated with control of serious bleeding in patients taking a common class of anticoagulants known as factor xa inhibitors, according to interim clinical trial results presented at the american college of cardiology’s 67th annual scientific session. After 20 years of discovery research, these agents are already licensed for several indications. Factor xa (fxa), a key serine protease that converts prothrombin to thrombin in the coagulation cascade, is a promising target enzyme for the prophylaxis and treatment of thromboembolic diseases.
Limits progression of bleeding within 12 hours of dose (onset as early as 1 hour) expensive: Total plasma clearance values were 884 and 1212 ml/h/kg in rats and dogs, respectively. And sales of each drug.
After 20 years of discovery research, these agents are already licensed for several indications. Drug names for factor xa inhibitors include: Coagulation factor xa was approved by the us food and drug administration (fda) on an accelerated basis.
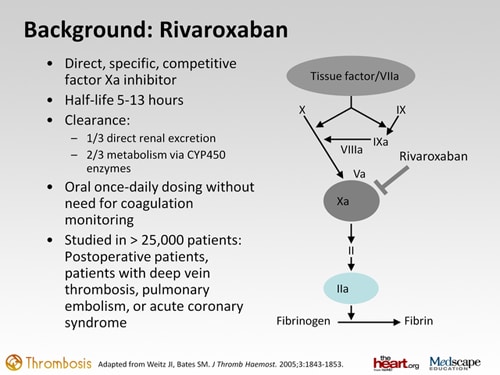
Andexanet is a recombinant modified factor xa molecule designed to bind to and disable factor xa inhibitors, thereby allowing factor xa produced by the body to play its normal role in the formation of blood clots. Factor xa represents an attractive target for antithrombotic drugs as blockade of factor xa permits inhibition of both the extrinsic and intrinsic coagulation pathways. The first xa inhibitor available on the market was rivaroxaban.
Darexaban maleate is a novel oral direct factor xa inhibitor, which is under development for the prevention of venous thromboembolism. Thus, the long path to finding replacements for warfarin has finally reached fruition. The factor xa inhibitors that are currently commercially available include rivaroxaban, apixaban, betrixaban, and edoxaban.
Factor xa inhibitors are preferred over lmwh and vka because they conveniently do not require injections every day compared to lmwh, their more predictable effects, lack of monitoring or frequent repeat doses, and fewer drug interactions compared to vka. The pharmacokinetics of ym466, a selective inhibitor for factor xa, was investigated after single intravenous and oral dosing to rats and dogs. [pmc free article] [google scholar]
Dosing, uses, side effects, interactions, patient handouts, pricing and more from medscape reference Fondaparinux is currently approved for treatment of dvt or pe, and vte prophylaxis after major orthopedic surgery or abdominal surgery. Andexxa or andexanet alfa (inactivated recombinant factor xa, to be released in 2018) antidote for eliquis or xarelto (rivaroxaban), but not other xa agents;
Fondaparinux is an indirect factor xa inhibitor, which acts by catalyzing factor xa inhibition by antithrombin. The analysis covers factor xa inhibitor market uptake by drugs; A factor xa inhibitor used to treat deep vein.
These drugs bind to factor xa and prevent the formation of thrombin by interrupting the extrinsic and intrinsic coagulation cascades. Fondaparinux is a synthetic indirect inhibitor of factor xa. Coagulation factor xa is used to treat uncontrolled bleeding in people who take the anticoagulants rivaroxaban (xarelto) and apixaban (eliquis).
This article focuses on rivaroxaban, apixaban, and edoxaban, the oral factor xa inhibitors in the most advanced stages of development. Potentiates the rate of neutralization of factor xa by antithrombin and, unlike heparin, does not inactivate thrombin4 Factor xa inhibitor drugs uptake helps in understanding the drugs with the most rapid uptake, reasons behind the maximal use of new drugs, and allow the comparison of the drugs on the basis of factor xa inhibitor market share and size which again will be useful.
$25,000 to 50,000 per patient Therefore, development of the oral factor xa Rivaroxaban, apixaban, and edoxaban, the oral factor xa inhibitors in the most advanced stages of development.
Factor x is activated, by hydrolysis, into factor xa by both factor ix (with its cofactor, factor viii in a complex known as. Its chemical structure is based on the natural pentasaccharide contained within heparin and lmwhs3; Thus, the long path to finding replacements for warfarin has finally reached fruition.
5 an earlier systematic review reported differences between factor xa inhibitors and standard. Mendell j, zahir h, matsushima n, et al. Darexaban glucuronide was the major component in plasma after oral administration of darexaban to humans.
Rivaroxaban, which inhibits activated factor x (xa), is currently in clinical trials and is the most advanced factor xa inhibitor. In clinical studies, healthy volunteers responded to this medicine, but further studies are.