
0, 2, and 6 months. 6 months after the first dose individuals are encouraged to adhere to the 0, 2, and 6 months vaccination schedule.
At elected date second dose:
Gardasil 9 vaccine schedule. At elected date second dose: Cervical, vulvar, vaginal, and anal precancerous or dysplastic lesions caused by hpv types 6, 11, 16, 18, 31, 33, 45, 52, and 58;. The second dose should be administered at least one month after the first dose and the third dose should be administered at least 3 months after the second dose.
Gardasil 9 vaccine is given in a series of 2 or 3 shots. Gardasil 9 protects against 9 types of hpv: Gardasil and gardasil 9 should be administered as a 0.5ml dose.
This study looked at the development of antibodies one month after the Gardasil 9 is a vaccine (injection/shot) given to individuals 9 through 45 years of age to help protect against diseases caused by some types of human papillomavirus (hpv). The aim of the immunisation programme is to provide the hpv vaccine (gardasil 9) schedule, as recommended in the immunisation guidelines for ireland within the academic year.
0, 2, and 6 months. Administer gardasil 9 as follows: Gardasil 9 is a vaccine used in males and females from the age of nine years to protect against the.
The recommended schedule is 2 doses given 6 to 12 months apart. The second shot should be given 2 months after the first shot and the third shot should be given 6 months after the first shot. Between them, types 16 and 18 are the cause of most cervical cancers in.
If you are from 9 to and including 14 years of age at time of first injection. If the second vaccine dose is administered earlier than 6 months after the first dose, a third dose should always be administered. Gardasil 9 is intended for adolescents and adults from 9 years of age onwards.
Safety and effectiveness of gardasil 9 have not been assessed in individuals older than 26 years of age. Gardasil®9 therefore extends the protection against disease caused by hpv. Each dose of hpv vaccine (gardasil 9) is 0.5 ml, administered intramuscularly.
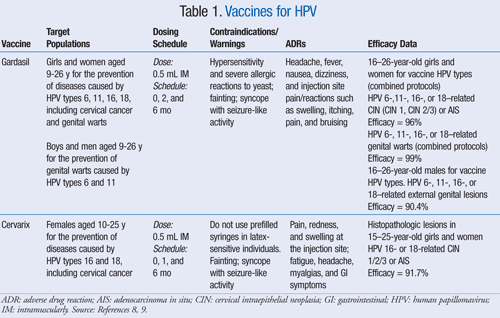
Gardasil®9 includes the hpv types covered by ® (6, 11, 16 and 18) plus an additional five oncogenic hpv types (31, 33, 45, 52 and 58). Gardasil 9 is a vaccine indicated in females 9 through 45 years of age for the prevention of cervical, vulvar, vaginal, anal, oropharyngeal and other head and neck cancers caused by human papillomavirus (hpv) types 16, 18, 31, 33, 45, 52, and 58; Gardasil 9 is given as an injection by your doctor.
3 vaccine dose is administered earlier than 5 months after the first dose, a third dose should always be administered. It protects against cancer of the neck of the womb (cervical cancer). Individuals 15 years of age and older at time of first injection.
0, 2, and 6 months. Registered for use in females aged 9 to 9</strong> to vaccine</strong> containing the major capsid (l1) protein of hpv types 6, 11, 16, 18, 31, 33, 45, 52 and 58. If the second dose is given earlier than 5 months, a third dose should be given.
2 months after the first dose third dose: Age regimen schedule * 9 through 14 years Vaccination might also decrease wart recurrence in men with frequent recurrences.
6, 11, 16, 18, 31, 33, 45, 52 and 58. You may have the first shot at any time as long as you are between the ages of 9 and 45 years. In the united states, the fda approved administration of the gardasil vaccine to males between ages 9 and 26 in 2009.
Vaccination with gardasil 9 may not result in protection in all vaccine recipients. The two doses of gardasil®9 should be administered 6 to 12 months. Individuals less than 15 years of age
The schedule for number of doses depends on age: The fda approved administration of the gardasil 9 vaccine to males between ages 9 and 15 in 2014, and extended the age indication, by including males between ages 16 and 26, in 2015. Scheduling appointments for hpv vaccination and wart treatment and std testing at new york urology specialists
The second dose is given 2 to 6 months after your first shot. Vaccination is also offered to men who have sex with men, up to the age of 45 years. 6 months after the first dose individuals are encouraged to adhere to the 0, 2, and 6 months vaccination schedule.
Gardasil 9 can be administered according to a. 0, 6 to 12 months. A third dose may be given 6 to 12 months after your first shot.
Sometime during the 2021 to 2022 academic year, the hpv vaccine used in the nhs programme will switch to gardasil 9.