Remdesivir is an experimental antiviral drug that was developed by us pharmaceutical company gilead sciences first for hepatitis c and then as a potential treatment for ebola and marburg virus infections. It is a form of viral hepatitis transmitted in infected blood.
Delivered 3 times a week.
Hep c drug trials. Occasionally a fever, dark urine, abdominal pain, and yellow tinged skin occurs. Completion of the initial phase (phase 1a. Although people who inject drugs (pwid) having the highest incidence and prevalence of hepatitis c virus (hcv) in the us, hcv treatment is rarely provided to pwid due to assumptions about poor adherence and reinfection risk.
Main digest the first clinical trials have started on a new investigational drug, discovered by researchers at cardiff university, which is being developed to treat infections caused by hepatitis c virus. The mean age of 277 participants was 37.2 (± 8.9) years, 62.5% were male, and 46.4% were caucasian (table 1). Talk with your liver specialist to see if you’re eligible for upcoming hep c clinical trials.
Clinical trials have started on a new drug which is being developed to treat infections caused by hepatitis c virus. Ezetimibe is currently being used as a treatment to lower cholesterol. Gilead submitted a new drug application for sovaldi to us food and drug administration (fda) in april 2013.
Centerwatch patient and professional information on clinical trials available across the u.s., with study descriptions and contact information. It is a form of viral hepatitis transmitted in infected blood. These trials study the safety and efficacy of certain antiviral medications in the.
Hepatitis c is an infectious disease caused by the hepatitis c virus (hcv) that primarily affects the liver; National institutes of health�s database of clinical trials for numerous medical conditions, including viral hepatitis. Delivered 3 times a week.
On average, participants reported they had used either heroin or cocaine for 16.2 (± 8.6, median = 17) years. This clinical trial, run by eiger biopharmaceuticals, will test the new drug lonafarnib in combination with other treatments. 76 rows drugs used to treat hepatitis c the following list of medications are in some way.
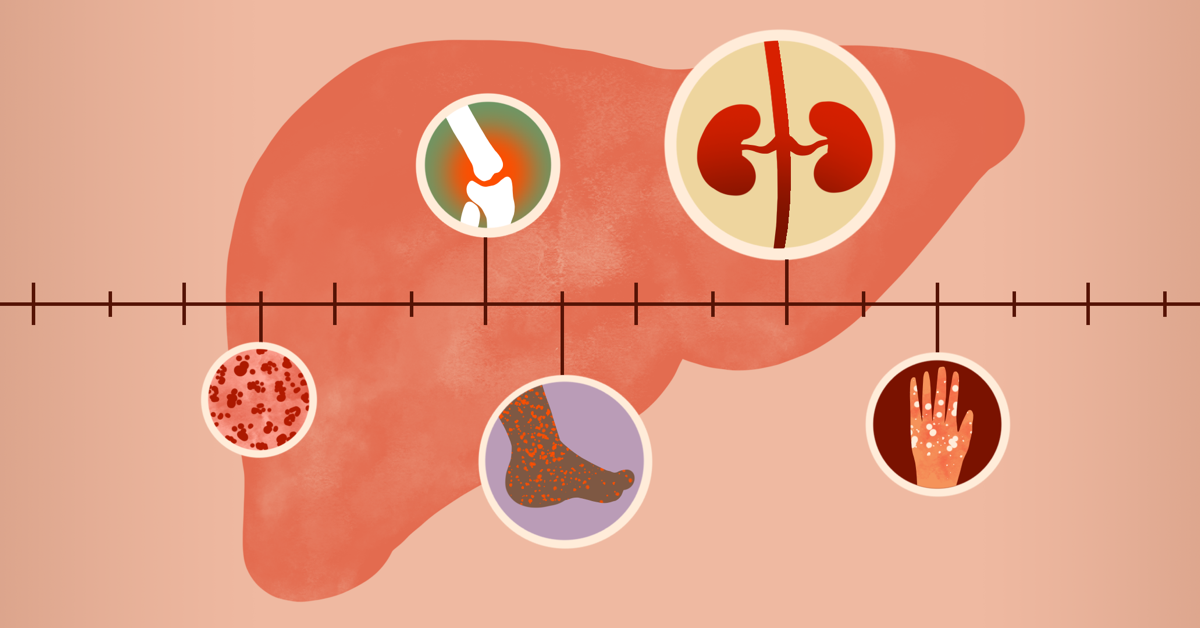
The trial involves transplantation of a deceased donor’s kidney infected with the hepatitis c virus into a recipient who does not have hepatitis c, and then curing the virus. The virus persists in the liver in about 75% to 85% of those initially infected. The following are selected studies supported by niaid and recruiting people with hepatitis c.
Sovaldi (sofosbuvir), developed by gilead sciences (gilead), is an oral nucleotide analogue inhibitor indicated for the treatment of chronic hepatitis c (chc) infection. Now being studied for effectiveness against hepatitis delta, it works by inhibiting ntcp, the receptor required for hepatitis delta to enter and infect liver cells. Not all patients with chronic hepatitis b, c, or d need to be on antiviral treatment.
Volunteers may be compensated for time and travel. The first clinical trials on a new investigational drug being developed to treat infections caused by hepatitis c virus have been successfully completed. Hepatitis c, or hcv, is caused primarily by sharing drug needles or is transferred through birth, and is curable.
2 for the purposes of this guidance, references to drugs and drug and biological products include drugs approved under section 505 of the federal food,. You can help niaid advance hepatitis research by volunteering to participate in a clinical study. Some patients may be monitored initially with treatment started later if the virus becomes more severe or if there is liver damage.
Many of these trials involve combinations of oral drugs and the course of treatment may be as. During the initial infection people often have mild or no symptoms. It is a type of viral hepatitis.
The latest medications for hepatitis c are taken by mouth, in pill form, and they have fewer side effects than previous options. Combining drugs used to treat hepatitis c with remdesivir boosted the effectiveness of remdesivir tenfold in cells. First diagnosed in 1989, the hepatitis c virus (hcv) is a significant public health problem affecting 185 million people worldwide.
Most patients (80% to 85%) who become acutely infected cannot clear the virus. Just how drastically is the market for hepatitis c drugs shrinking? The drug was one among four therapies.
The situation is so bad that less than three years after shelling out $3.9 billion to. Injecting risk behaviours following treatment for hepatitis c virus infection among people who inject drugs: The australian trial in acute hepatitis c.
Remdesivir is an experimental antiviral drug that was developed by us pharmaceutical company gilead sciences first for hepatitis c and then as a potential treatment for ebola and marburg virus infections.