In the united states, treatment with recombinant products is the standard of care. Recombinant factor concentrate is made in a special way.

The cumulative risk for patients treated with a single recombinant product was reported to range from 36.0 29 to 38.7% 30.
Recombinant factor viii products. However, it is unknown whether this advantage translates into. This concentrate is genetically engineered using dna technology. Table 1 licensed recombinant factor viii products note:
The development of recombinant factor viii (rfviii) was initially driven by the necessity to treat hemophilia a (ha) patients with fviii concentrates without the risk of transmitting infectious agents. The final product vials will be packaged with the following: ‐8°c, room up to 25°c for up to 12 licensed, distributed in all provinces except quebec comments full‐length factor viii molecule, monoclonal mouse antibody used in factor viii purification procedure product albumin as stabilizer human protein in.
Factor viii is activated proteolytically by a variety of coagulation enzymes, including thrombin (f2; The development and introduction of recombinant factor viii (rfviii) concentrates nearly 20 years ago represented a major advance in the treatment of hemophilia a patients. Choice of recombinant factor viii product.
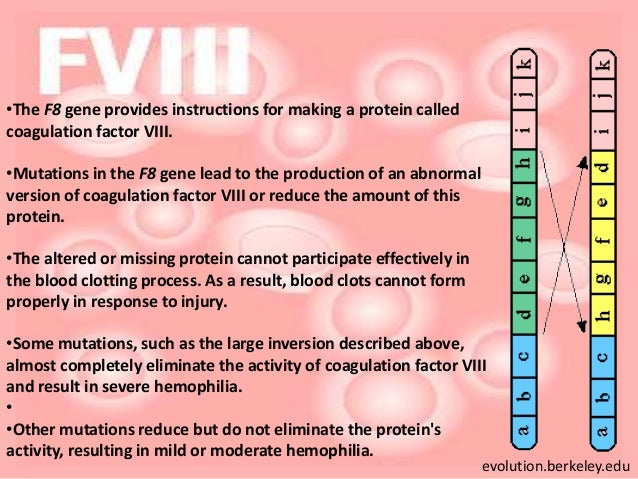
Factor viii is produced in liver sinusoidal cells and endothelial cells outside the liver throughout the body. Scientists learned how to turn certain animal cells into “factories” to make human clotting factor proteins. Hemophilia a is a bleeding disorder caused by congenital deficiency of factor viii (fviii), a protein found in blood.
More recent surveys found that the incidence of inhibitors ranged from 16.7 31 to 32% 32 in patients treated with a second generation recombinant fviii product. Commercially prepared factor concentrates are treated to remove or inactivate bloodborne viruses. The cumulative risk for patients treated with a single recombinant product was reported to range from 36.0 29 to 38.7% 30.
Recombinant factor viii manufacturing process which does not incorporate any human blood derivatives. Discover advate® [antihemophilic factor (recombinant)] , the most widely used factor viii product. Some recombinant factor viii products have human albumin (a body protein) added to them as a stabilizing agent.
It has the same effects as human factor viii, so it can be infused to prevent or treat bleeding. These products are not made from human blood. Required dose (iu) = body weight (kg) x desired factor viii rise (iu/dl or % of normal) x 0.5 (iu/kg per iu/dl) dosing examples for standard recombinant factor viii products:
The product must be administered intravenously. 162 safety aspects of factor ix products include viral safety, immunogenicity and other adverse events. Join leading researchers in the field and publish with hindawi.
For patients with hiv infection, the use of recombinant products has been associated with a slower decline in cd4 cell count, and many experts recommend using recombinant products in that setting. Recombinant factor products are made in a laboratory using recombinant technology. Although inhibitors can still occur, the risk of infection will be reduced by a recombinant factor viii.
Six recombinant factor viii (rfviii) products have been marketed worldwide. In the united states, treatment with recombinant products is the standard of care. Recombinant factor concentrate is made in a special way.
Comparing factor use and bleed rates in u.s. The dose is determined by calculating how much factor viii has to be given to raise. The factor viii in this product is synthesised using chinese hamster ovary cells.
Factor viii is a recombinant product and not a plasma derived factor concentrate. Recombinant factor concentrate is made in a special way. Scientists learned how to turn certain animal cells into factories to make human clotting factor proteins.
Recombinant factor viii, ix, and viia products have been available in the united states since the 1990s. 42 while the use of. Food and drug administration (fda) approved recombinant factor viii (8) concentrate, which does not come from human plasma.
It has over 15 years of treatment experience. Thrombogenicity should also be considered a potential safety 165 The human gene for the desired factor is placed in the animal cells which begin.
Over the last three decades the safety of rfviii has.