
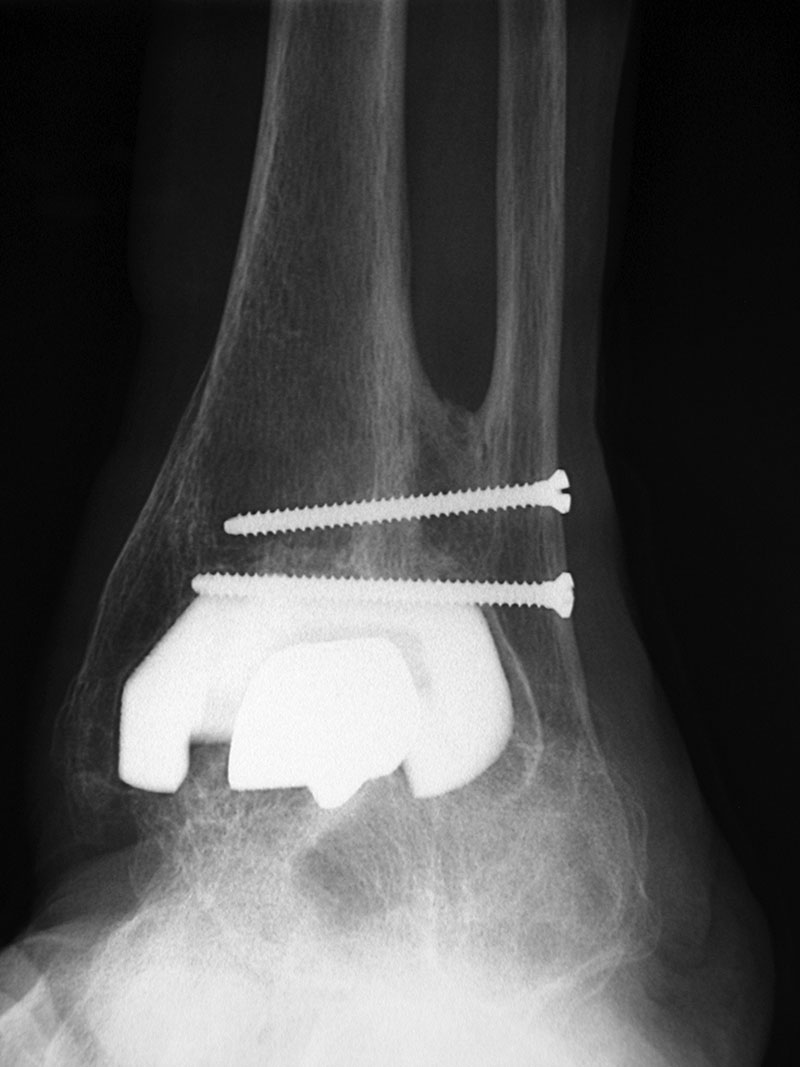
Ankle arthroplasty is increasingly used to treat advanced ankle arthritis. Prior to star, surgeons had limited options when performing a total ankle arthroplasty.
The 65 patients (34 women and 31 men;
Scandinavian total ankle replacement. Mobile bearing ankles have been available in europe for several years, but the fda had been concerned with the safety and efficacy of star prior to its approval. The purpose of the current prospective study was to determine the midterm results of 68 total ankle replacements with the scandinavian total ankle replacement (s.t.a.r.) prosthesis. For the first two weeks after surgery it is normal to have a moderate amount of pain.
Clinical outcomes were measured with the. The purpose of this study was to review the history of the implant, describe its design, highlight important aspects of its surgical technique, and assess the available results. The scandinavian total ankle replacement (star) system.
The purpose of this study was to evaluate survival and clinical outcome of the scandinavian total ankle replacement (star) prosthesis after a minimum of ten years up to a maximum of 19 years. The purpose of this study was to review the history of the implant, describe its design, highlight. You may need to use pain medicine(s).
Ankle arthroplasty is increasingly used to treat advanced ankle arthritis. Star (scandinavian total ankle replacement) surgery is a procedure designed to help relieve ankle pain while still allowing you to move your ankle normally. The star was approve d by the fda on may 27, 2009.
Clanton and haytmanek offer the star ankle replacement for individuals suffering from severe ankle arthritis. The 65 patients (34 women and 31 men; The scandinavian total ankle replacement (star) is one of the most commonly implanted total ankle replacements globally.
Patients were followed both radiologically and clinically; “total” means that your entire ankle joint will be replaced. The 65 patients (34 women and 31 men;
The most common complications include delayed wound healing, and lateral and medial malleolar fractures, with their incidence decreasing along with the rising surgeon�s. This pain may slowly decrease over time, but it is not unusual to experience some discomfort for up to three months and swelling may continue for up to a year after surgery. The star has been in clinical use in.
However, early and midterm data have demonstrated that the scandinavian total ankle replacement (star) or agility total ankle may be a reasonable alternative to an ankle joint fusion share this add to bookmarks 24 the survival rate for 12 years was 70%. The concept was to mobilize, align, and stabilize the ankle before resurfacing it.
It is the only implant approved by the food and drug administration (fda) for use in the united states without cement and therefore relies on bony ingrowth into its. Prior to star, surgeons had limited options when performing a total ankle arthroplasty. Our preliminary results with the scandinavian total ankle replacement (star) are encouraging.
Since the late 1800’s, severe ankle arthritis has been treated by fusing the bones of the ankle together to eliminate the arthritic joint. The purpose of the current prospective study was to determine the midterm results of 68 total ankle replacements with the scandinavian total ankle replacement (s.t.a.r.) prosthesis. Scandinavian total ankle replacement ankle replacement outcomes have been analysed in numerous studies.
Common side effects from total ankle replacement surgery: Between 1997 and 2002, 34 consecutive ankles were replaced with the star ankle prosthesis and reviewed in 2016. We retrospectively reviewed 45 patients (52 ankles) who had primary total ankle replacements using star prostheses, in order to assess survivorship.
The star (scandinavian total ankle replacement): We think that these problems can be solved by some improvements in the instrumentation and we therefore propose some modifications and technical tips we learned during our first 23 cases.