5 8 9 however, little is known about the characteristics of contemporary phase iv clinical trials and whether these studies are of sufficient quality. Dose refinement:evaluation of newformulations,dosages, durations oftreatment.
Following fda approval, a treatment goes through phase 4.
Phase 4 clinical trial. Temasek analytical services facility 1.4.2. Generally, this phase has been conducted in order to analyze the safety, tolerance, pharmacodynamic and A phase i trial tests an experimental treatment on a small group of often healthy people 20 to 80 to judge its safety and side effects and to find the correct drug dosage.
Phase iv trials are often used to conduct continued monitoring beyond initial drug development and regulatory approvals, but may still be subject to clinical trial limitations with respect to. Dose refinement:evaluation of newformulations,dosages, durations oftreatment. To determine a safe dosage range and identify side effects).
What is a phase 4 clinical trial? Such trials, involving thousands or tens of thousands of enrollee patients, are either mandated by regulatory authorities or undertaken voluntarily by a pharmaceutical manufacturer once the. 5 8 9 however, little is known about the characteristics of contemporary phase iv clinical trials and whether these studies are of sufficient quality.
Detect the unknown/rare adverse drugreaction/s. Phase 4 clinical trials begin once the drug is marketed. Knowing the phase of the clinical trial is important because it can give you some idea about how much is.
What are the different types of clinical trials? Following fda approval, a treatment goes through phase 4. It can last for several years as researchers continue to monitor the efficacy and safety of the treatment.
Refine, extend, minimise and modify the therapeutic indications of the product; By definition, an nis is a study conducted to assess safety, tolerability and effectiveness of marketed medicines in clinical practice, i.e., in a naturalistic setting where choice of therapy is consistent with approved prescribing information (no study drug to be supplied) and in line with current practice at the study site; Each phase is designed to answer certain questions.
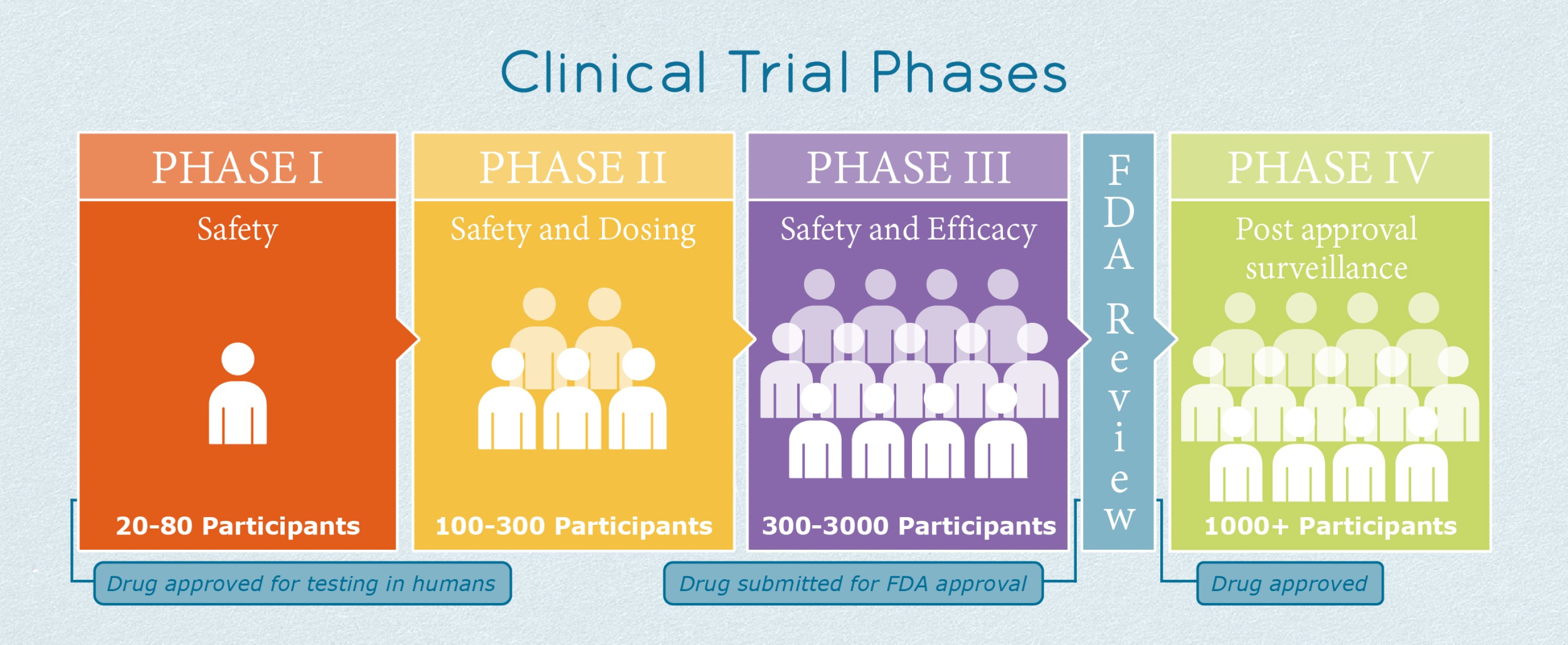
Address temasek polytechnic, school of applied sciences. This phase involves the largest group of participants. Clinical trials are usually conducted in phases that build on one another.
The number of people participating in clinical studies grows along with our understanding of the investigational medicine, and the research. The third phase of successful clinical trials is referred to as phase i trials that accounts for the first step for volunteer testing. Often clinical trials do not fit neatly into one of the four phases described above, and it may not be that simple to discern which phase a trial comes under.
Phase 4 clinical trial means a product support clinical trial of a product that is commenced after receipt of maa approval in the country where such trial is conducted. Address of the trial site t&t family health clinic and surgery. Evaluate the risk and effectiveness of the treatment of.
Small phase iv trials might be used to evaluate the effectiveness of a given drug in a special patient subgroup, or in special situations.5 however, our study included only phase iv trials with ‘safety’ as an end point and most of these trials (77.6%) had an enrolment of <300. Clinical trials of biomedical interventions typically proceed through four phases. Phase i clinical trials are done to test a new biomedical intervention for the first time in a small group of people (e.g.
The process of learning about and developing an investigational medicine is divided into four phases. In the phase iv trials with safety as the primary end point, the average sample size was only 104. At first, very few people receive the medicine being studied.
A phase 4 clinical trial begins after a drug has been approved for use in the general population following phase 1 2 and 3 trials to rigorously test its efficacy and safety. Phase 3 is the final phase before a treatment receives fda approval. Confirm the efficacy and safety profile inlarge populations during practice.
It has several objectives, namely: Telephone number of the trial site: What rights do participants have in a clinical trial?
The four phases of clinical trials. The definitions of the four phases of clinical trials are not distinct and there is some overlap between them. For at least the entire time a treatment* is on the market, the fda monitors for public safety and potentially serious adverse events.
The aim of the above trial was to establish the effectiveness of melatonin.